While we do not have the full study results as this is only an interim analysis the data demonstrated that the study vaccine can prevent COVID-19 disease in adults who. This is an event-driven trial that is set to enrol up to 30000 subjects aged 18 to 85 years.
 Safety And Efficacy Of The Bnt162b2 Mrna Covid 19 Vaccine Nejm
Safety And Efficacy Of The Bnt162b2 Mrna Covid 19 Vaccine Nejm
21720 received BNT162b2 and 21728 received placebo Figure 1.
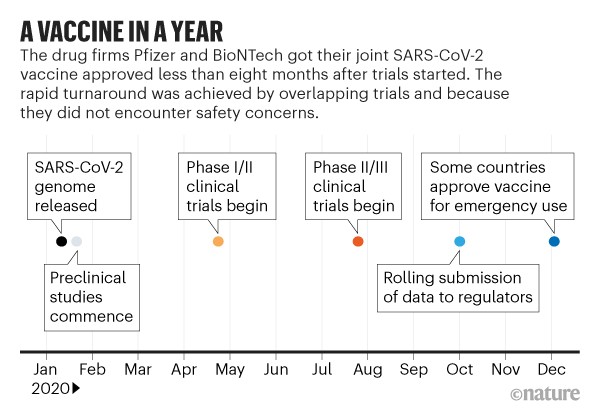
Pfizer clinical trial data. Clinical trial data should be accessible and transparent Pfizer believes that it is important for researchers trial participants regulators and others acting in the best interest of patients to have access to clinical trial information to advance medical understanding and progress. At the data cut-off date of October 9 a total of 37706 participants had a. Pfizer and BioNTech recently have advanced another Covid-19 vaccine candidate BNT162b2 into a Phase IIIII clinical trial.
Ad Search Clinical Trial Data. December 29 2020 - Pfizer and BioNTech recently announced additional data on neutralizing antibody and T cell responses from the Phase 12 clinical trial of its COVID-19 vaccine candidate in Germany. April 9 2020 The machine learning ML capabilities of four companies were put to the test in a first-of-its-kind hackathon organized by Pfizer late last year where the singular goal was to see whether artificial intelligence AI could predict and identify data discrepancies from datasets of 30 completed clinical trials.
Get Results from 6 Engines at Once. The Pfizer and BioNTech covid-19 vaccine may provide some early protection starting 12 days after the first dose the peer reviewed results of a phase III trial have found. Get Results from 6 Engines at Once.
Pfizer Inc and BioNTech said new clinical trial data signalled that their COVID-19 vaccine could protect against the new coronavirus variant first discovered in South Africa. The companies continue to capture data from the Phase III trials and expect to submit findings on BNT162b2 in the near future. Specifically researchers reported that all 37 participants vaccinated with BNT162b2 showed a broad immune response with SARS-CoV-2-specific.
By age group 887 in the younger group aged 18 to 55 years and 797 in the older group. The study published in the New England Journal of Medicine 1 found that vaccine efficacy between the first and second doses was 52 95 credible interval 295 to 684 with 39 cases of covid-19 in the vaccine group. In addition to the feasibility of applying AI to clinical data the competition demonstrated.
The Phase 3 clinical trial of BNT162b2 began on July 27 and has enrolled 43661 participants to date 41135 of whom have received a second dose of the vaccine candidate as of November 13 2020. The FDA analysis of data from Pfizers large-scale clinical trial involving roughly 44000 people affirmed the Manhattan-based drugmakers finding that the shot was 95 percent effective at. To reimburse participants for clinical trial-related expenses such as transportation.
To incorporate as much patient input as possible into the design of our clinical trials. Among all study vaccine recipients asked to complete diaries of their symptoms during the 7 days after vaccination 847 reported at least one local injection site reaction. A total of 43448 participants received injections.
Clinical trials for the Pfizer-BioNTech vaccine included people from the following racial and ethnic age and sex categories. To ensure that diverse communities have the opportunity to participate in our clinical trials. Dear Participant Today Pfizer and BioNTech announced the results of the first interim analysis from our COVID-19 vaccine study.
To make clinical trial results available to. Clinical Trial Demographic Information. 44 Asian.
Ad Search Clinical Trial Data. The shot was 100. In the phase 23 evaluable efficacy data submitted to the FDA the Pfizer trials population includes 37796 patients of whom 37088 981 completed the two-dose regimen of either BNT162b2 or placebo split 11.
Clinical Trial Data and Results At Pfizer we believe researchers trial participants regulators and others acting in the best interest of patients should have access to clinical trial data to advance medical understanding and promote data transparency. CNNClinical trial results of PfizerBioNTechs Covid-19 vaccine showed its efficacy is 100 and it is well tolerated in youths ages 12 to 15 the companies said Wednesday. At the time of EUA submission just 13 patients had withdrawn from assessment due to an adverse event.
