In the measles mumps and rubella MMR and varicella VAR rows pregnancy column an asterisk was added to indicate MMR and VAR vaccines should be administered after pregnancy. The Vaccine Schedule is an ECDC tool and interactive platform of vaccination schedules for individual European countries and specific age groups.
 Immunization Schedule For Children Birth Through 6 Years Wic Works Resource System
Immunization Schedule For Children Birth Through 6 Years Wic Works Resource System
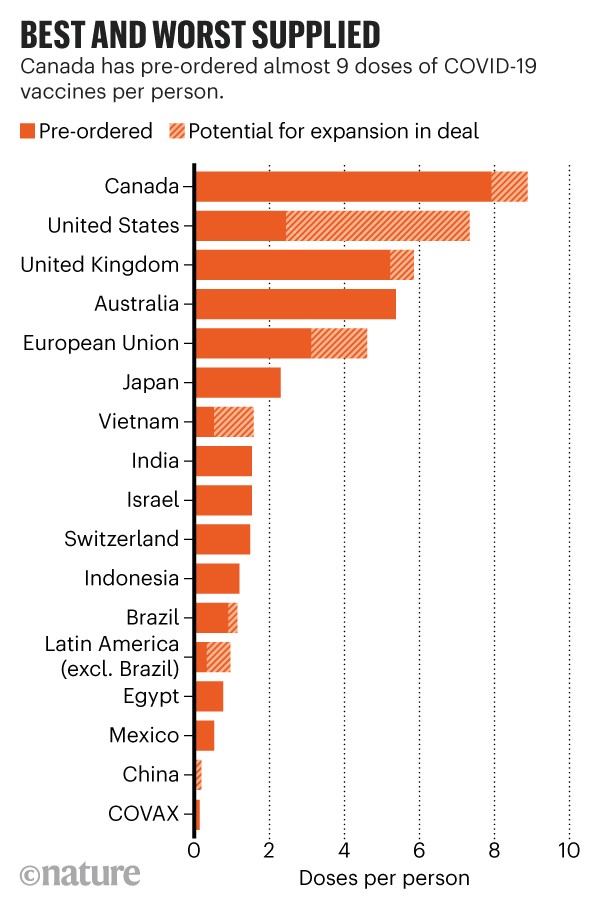
Two doses of HepA vaccine are needed for lasting protection.

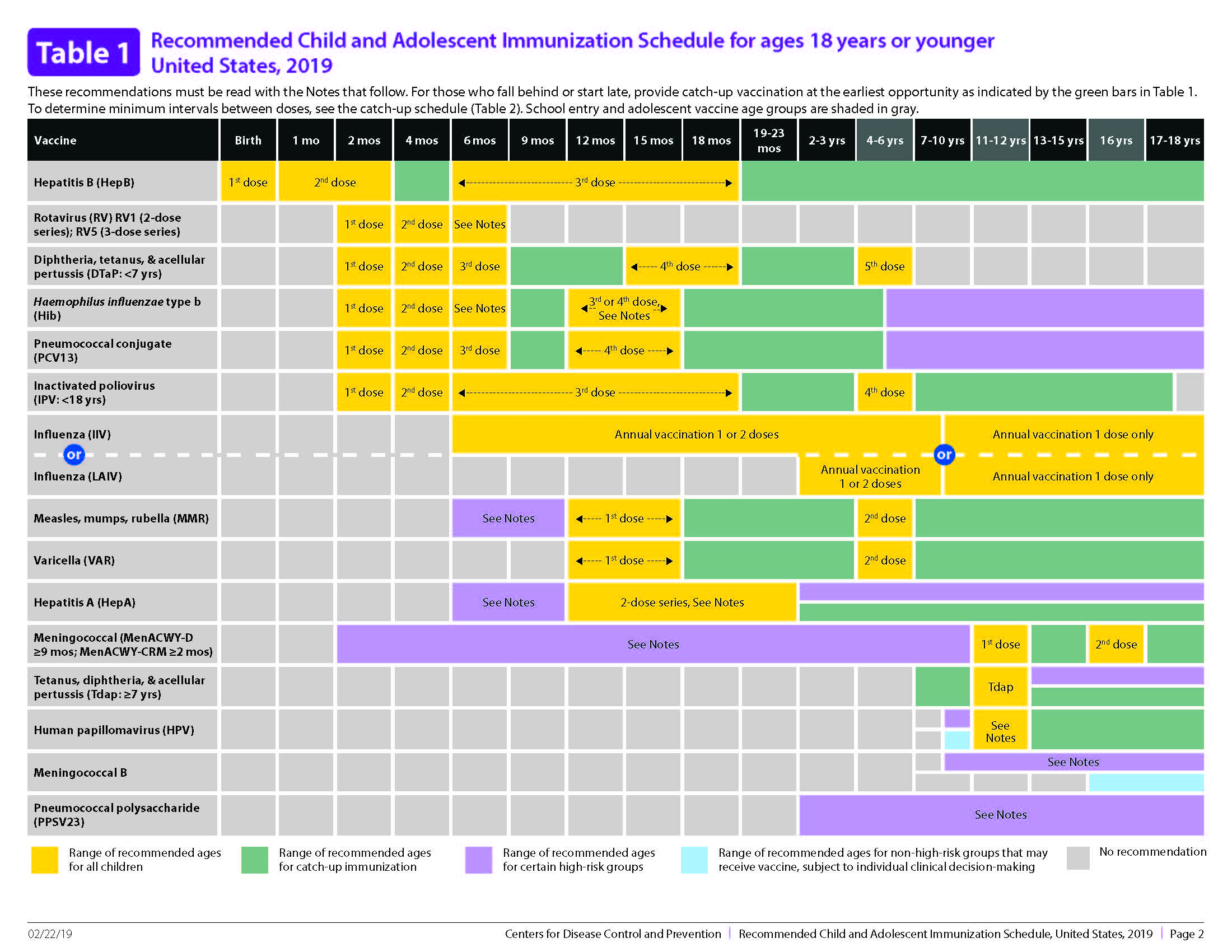
Pediatric vaccine schedule. Table 3 Recommended Child and Adolescent Immunization Schedule by Medical Condition. Any dose not administered at the recommended age should be administered at a subsequent visit when indicated and feasible. The result is that the vaccine schedule has become more complicated than it once was and children are receiving far more shots than before see Vaccine Safety for answers to the questions.
Giving insight in the vaccine schedules in all countries of the EU provided by the European Centre for Disease Prevention and Control ECDC. Birth - 6 Years. Since the mid-1980s many vaccines have been added to the schedule.
If you are 16 or 17 years of age and meet the eligibility requirements listed above you can schedule your first vaccine dose online via web browser or by using an App on your smartphone. Schedule online or via App. Four doses of the Haemophilus influenzae type b vaccine are given.
Vaccination schedule Vaccinations arent all given right after a baby is born. Slight variations in this schedule are possible. Now children could receive as many as 27.
2021UNITED STATES Vaccines in the Child and Adolescent Immunization Schedule Vaccines Abbreviations Trade names Diphtheria tetanus and acellular pertussis vaccine DTaP Daptacel Infanrix Diphtheria tetanus vaccine DT No trade name Haemophilus influenzae type b vaccine Hib PRP-T Hib PRP-OMP. H-B-Vax II Paediatric or Engerix B Paediatric. However older children may not need all the vaccine doses or may need different vaccines.
Childhood vaccination also see influenza vaccine Birth. Vaccination schedules for individual European countries and specific age groups. Your pediatrician will discuss these issues with you.
Parent-Friendly Schedule for Infants and Children birth-6 years Parent-Friendly Schedule for Preteens and Teens 7-18 years Resources for Parents. National Immunisation Program Schedule from July 2020 Age. The following 2021 schedules indicate the recommended ages for routine administration of currently li censed vaccines for children and adolescents.
Just ask your GP general practitioner Should I delay taking my baby for their vaccines during Covid-19. Hepatitis B usually offered in hospital a. Influenza flu vaccine for the first time and for some other children in this age group.
All children and adolescents over 24 months of age. Birth 6 Years. One vaccination for varicella chickenpox no earlier than age 12 months and only if your child does not develop chickenpox on his or her own must be verified by a health care provider Three vaccinations for rotavirus a type of infection that causes severe diarrhea.
The first dose of HepA vaccine should be given between 12 months and 23 months of age. In the human papillomavirus. Following the recommended vaccine schedule provides your child with the best protection from potentially serious diseases.
The following schedules show the recommended ages for routine administration of currently licensed vaccines for children. 7 - 1 8 Years. The first at 2 months the second at 4 months the third at 6 months and the fourth at about 12 months of age.
The vaccines already given will still work and your child will still develop protection. In the LAIV row changed abbreviation to LAIV4. Any dose not administered at the recommended age should be given at a subsequent visit when indicated and feasible.
See which vaccines your child needs from birth through age 6 in this parent-friendly immunization schedule. Each is given on a different timeline. Download CDC Vaccine Schedules free for iOS and Android devices.
The second dose should be given 6 months after the first dose. 2 months can be given from 6 weeks of age. Do vaccines weaken the immune system and more.
7 18 Years. Theyre mostly spaced throughout the first 24 months of your childs life and.